The World Health Organization reported in the first days of May that to date more than 100 candidate vaccines [1] have been presented against the new coronavirus responsible for the pandemic more severe of the last decades, of which 7 or 8 are the most promising since they are those that are likely to deliver better results in terms of their ability to generate immunity in a preventive way.
But “creating” a vaccine is not as easy or as fast as it is, and there are reasons why it should be. Historically, the development of vaccines has taken many years, for example, for typhoid fever, a disease that became relevant in the 19th century, it was not until 105 years after the causative agent was identified. He managed to develop the vaccine capable of fighting the disease. For measles the process took more than 40 years and for HIV ―human immunodeficiency virus― 37 years without reaching a successful result [2] (Figure 1).
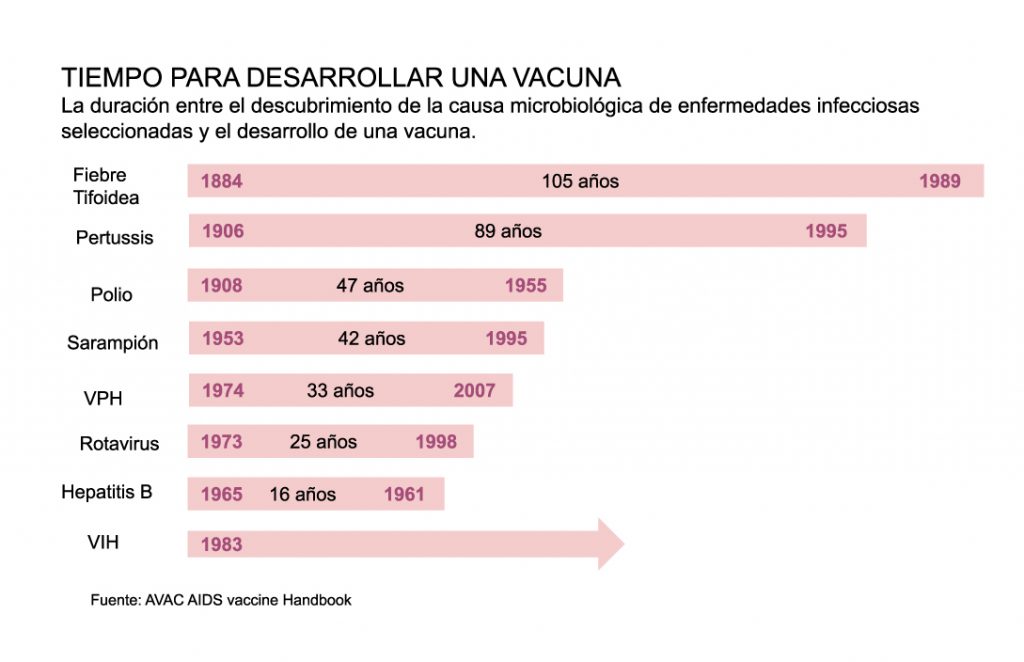
The mumps vaccine, considered ―for now― as the fastest in history , took 4 years from the collection of biological samples to approval and licensing in 1967. This disease is caused by a paramyxovirus type virus of which only one serotype [3] is known, a fact that facilitated the speed of the process. However, everything indicates that the vaccine for COVID-19 will arrive to remove the throne from the mumps vaccine within the next few months.

The Chilean experience
In our country, the vaccine against respiratory syncytial virus in which the research team of Dr. Alexis Kalergis , director of the Millennium Institute of Immunology and Immunotherapy (IMII) of the Pontificia Universidad Católica, began this effort in 2004 and the completion of this process is expected shortly [4] .
However, Dr. Kalergis’ team is one of many laboratories that has redirected their work to the development of a COVID-19 vaccine . The vaccine in development by this group consists of using coronavirus antigens or antigen fragments , a strategy they chose for its ability to induce a favorable immune response to eliminate the virus in the absence of excessive inflammation.
The use of antigens of SARS-CoV-2, which correspond to molecules -generally proteins outside of viruses- that are specifically recognized by the host’s immune system and trigger defense, is a strategy equivalent to that already implemented by this research group in the successful development of the vaccine against respiratory syncytial virus.
703/5000 According to Dr. Kalergis, the Chilean vaccine could be available for large-scale production in the minimum 2-year period, only if conditions are met in the best possible scenario . They recently received a financial contribution from the Copec-UC Foundation to continue with the vaccine trials, which undoubtedly means facilitating the process. However, the researcher is sensible to emphasize that “ these processes take time and require a series of preclinical and clinical studies that must comply with quite strict regulations” [5] sup >, referring to the beginning of human tests estimated for the year 2021.
Normal times against the urgency of the pandemic
Currently the development of vaccines continues to be a long, complex and linear process that takes an average of 10 to 15 years , but in this case for COVID-19 the normal deadlines have been modified and the This has been called a “accelerated development” , where research and regulatory process times are shortened, in addition to speeding up and even overlapping the experimental stages of preclinical and clinical trials (Figure 2). This adaptation does not mean that the safety or quality of the final product is reduced .
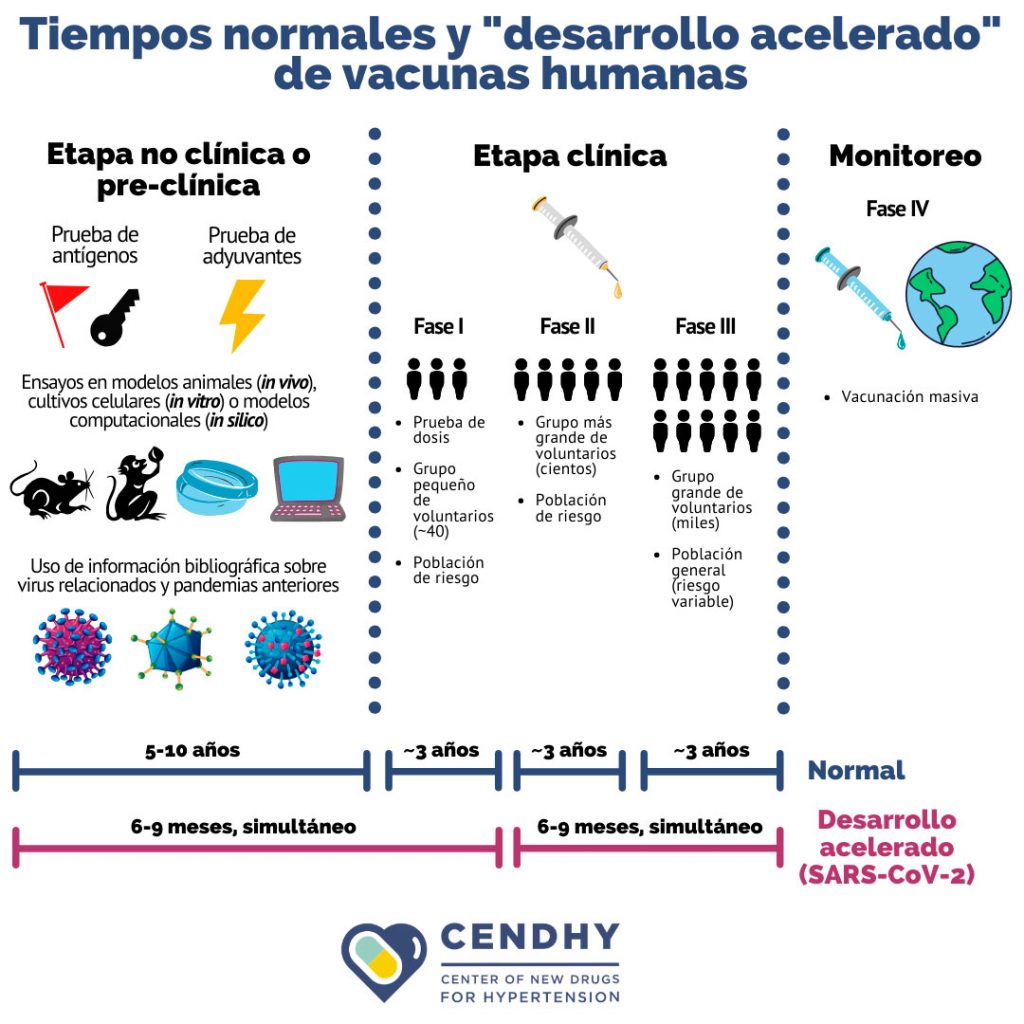
Along with efforts to contain the spread of the virus as well as sanitation and containment measures, hundreds – if not thousands – of scientific research teams around the world are focused on finding a vaccine to face the pandemic and immunize the population. Despite the fact that the hope of the public lies in a vaccine for COVID-19, researchers are clear that the development of a vaccine is not a speed race, but a true human pyramid that it requires the cooperation and commitment of many actors, and that it is equally important to be able to guarantee both the efficacy and the safety of a long-awaited prevention element such as this vaccine.
The urgency for a prompt solution
At the beginning of June, 6 million of confirmed positive cases for this coronavirus were recorded worldwide, and more than 371,000 deaths [6] sup have been reported >, reflecting the severity of the new pandemic that we face since the first case reported in the Chinese region of Wuhan in December 2019 [7] .
The disease caused by the new coronavirus, called SARS-CoV-2 , is named COVID-19 and produces flu-like symptoms: fever, dry cough, shortness of breath, muscle pain, and fatigue [8] . In severe conditions, it can lead to pneumonia, acute respiratory distress syndrome, including sepsis – exacerbated response to infection – and septic shock, leading some patients to death.
Why a vaccine?
First, another question needs to be answered: How does a vaccine work? And to clarify this question, we must first address certain points related to the human immune system , the defense system of our body, which is made up of specialized cells and various organs in our body.
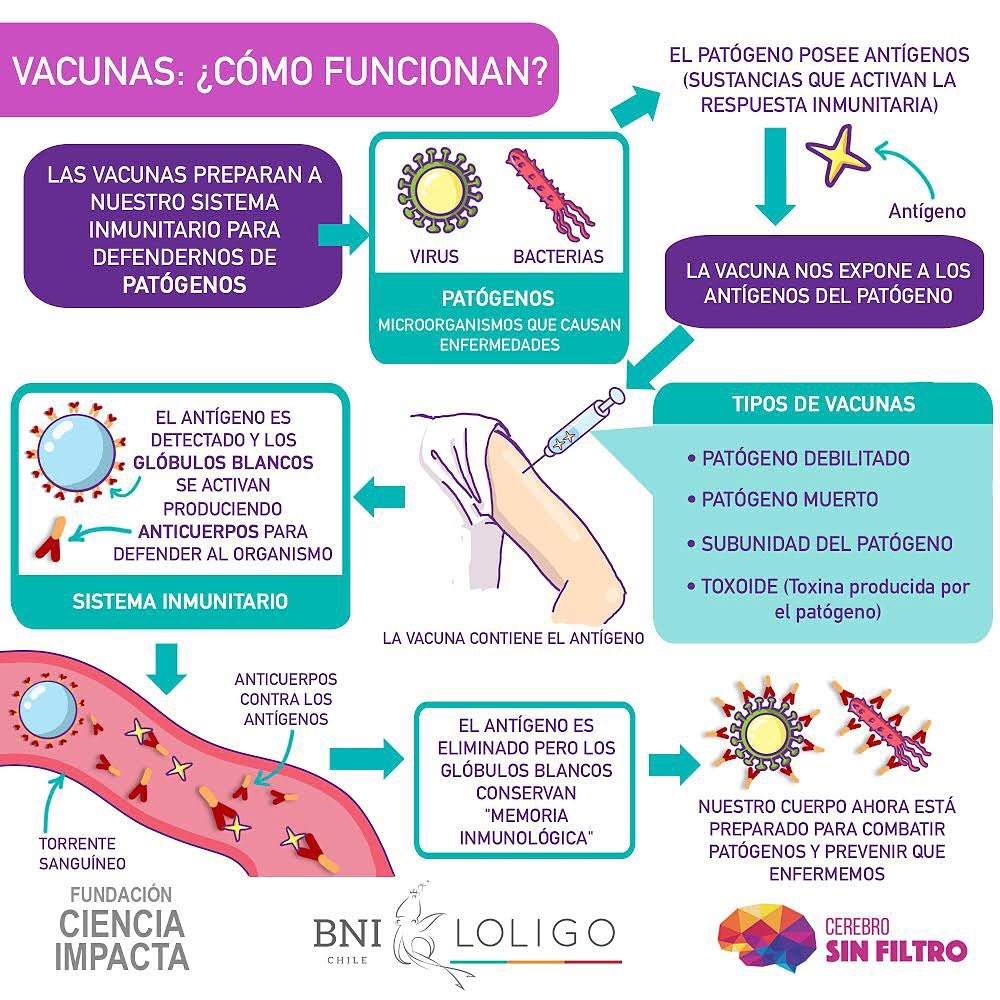
The objective of vaccination is clear: prevention . A vaccine simulates an infection but does not trigger disease, what it does generate in the body is the start of the immune response , that is why it refers to vaccination as part of the state programs immunization of the population [9] . The human immune system acts in coordination through the innate and adaptive responses, and vaccination targets the adaptive immune system, which “learns” to recognize unknown invading pathogens, such as SARS-CoV- 2, in order to prepare the body for a new event of infection by the same pathogen .
It is important to understand that a vaccine does not deliver the individual who receives the shields to protect themselves against the disease, it is also not a remedy that alleviates the disease. The way in which a vaccine provides protection is that it promotes the organism to generate – with its own raw material – its specific defenses against this infectious agent.
The route of the coronavirus in the human body
Faced with exposure to the new coronavirus, our body cannot identify it and freely “lets it pass” through various routes of entry: nose, mouth, skin [10] and any epithelium with mucosa that is orient abroad. SARS-CoV-2 is a virus whose genetic material is a individual strand of ribonucleic acid (RNA) that, unlike in human cells, is not protected within a closed compartment such as it is the cell nucleus. Another fundamental difference is that human genetic material is a double strand of deoxyribonucleic acid (DNA) . Despite this apparent incompatibility, this coronavirus is able to break into our cells, into the nuclei of our cells, into our genetic material (DNA) and cause great damage.
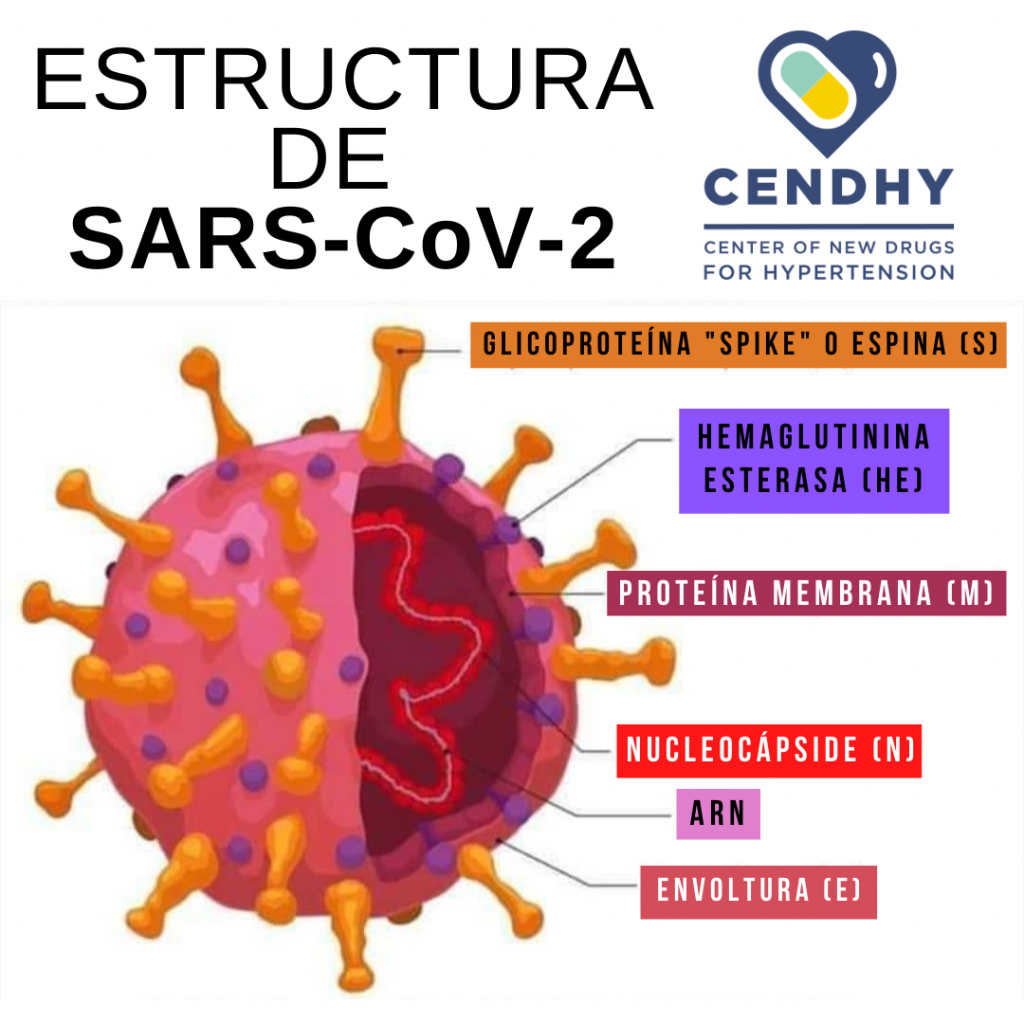
Although the RNA of this virus is not found within a nucleus, the coronavirus has an outer boundary known as envelope , made up mainly of lipids and proteins. Particularly important are the ―at this point― famous protein spike or “thorn” , and the M protein, both facing outward (Figure 3). These two proteins are mainly responsible for the entry of SARS-CoV-2 into the circulation, since the virus binds to Angiotensin II Converting Enzyme (ACE2) on the surface of human cells in numerous organs of the body, such as the lungs, kidneys and heart, and it enters without much problem.
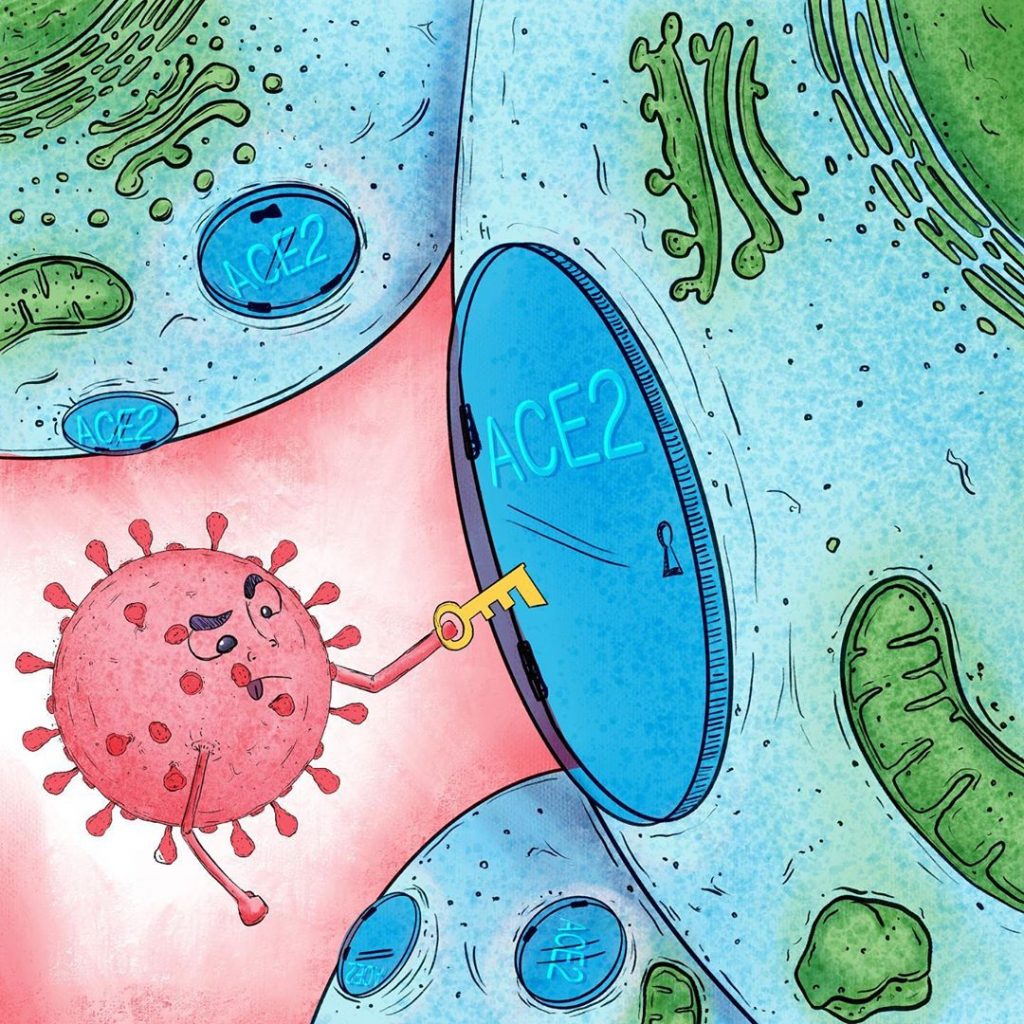
This process of recognition, binding and anchoring of ligands with their receptors is frequently described using the “key and lock model” (“ Lock and key ” [11 ] ), where the key is one of the virus proteins, in this case the thorn protein, and the lock is the surface protein, the ACE2 receptor, which allows access to the cells (Figure 4).
Once inside a human cell, the coronavirus takes control of all available tools and begins to produce more viral particles, clones of itself, and to infect both nearby and distant cells. The immune response begins when a specialized type of cell, Antigen Presenting Cells (APC), envelops the virus and they expose on their own surface some important fragments of this to “draw attention” to other cells of the immune system and thus activate them, that is why they are said to “present” them.
These cells that are “called” the APCs are “helper T cells”, which in turn allow other branches of the immune response to begin: B cells or B lymphocytes produce antibodies They prevent the virus from infecting more human cells, as well as “tagging” the virus to be recognized as a target for destruction. Thanks to this brand, “cytotoxic T cells” identify the cells infected by the virus and eliminate them, hence their name: “toxic to cells” (Figure 5). This is, in a simplified way, how the immune response is carried out against a new or unknown infectious agent.

Vaccination and immunological memory
vaccination mimics an infection as it employs these important elements of the virus, which we mentioned earlier, that “draw attention” to cells of the immune system, activate them and trigger the initiation of the immune response. The fundamental difference is that this imitation is in a controlled way and does not generate disease , however, once the infection imitation “disappears”, the body is left with a reserve of memory lymphocytes that will “remember” how to fight the disease in the eventual event that it is found in the future with the same infectious agent, which is known as immune memory (Figure 6). This memory is specific and can last for several years, it is reactivated each time the organism is again faced with the same pathogen.
Vaccine development: “not so easy, not so fast”
To develop a vaccine one must first identify the microbiological agent causing the disease, study it, characterize it, and then investigate the antigens that will be useful for a possible vaccine. An antigen is one of these “ important elements that attract the attention of cells of the immune system, activate them and these trigger the initiation of the immune response ” to which we have referred. The technical definition of antigen is “any substance that activates the immune response” [12] .
All vaccines are intended to expose an antigen to our body that will not cause disease, but will elicit an immune response that will block or eliminate the pathogen if the person is infected next time. Antigen presentation is the milestone that triggers the immune response.
After the research and development stage in the process to create a new vaccine, details such as its regulations should be reviewed, and then manufactured on a small scale with the corresponding quality control . The next step is preclinical tests in animal models ( in vivo ) to verify that an immunological reaction does indeed occur and that the vaccine is not harmful, to finally begin the clinical trials consisting of 4 consecutive stages: I, II, III and IV, in which vaccines are tested in humans.
Each stage must be duly passed and certified to advance to the next, so the total process of developing a vaccine is extensive in time, complex by the multiple control instances , and linear where the stages are consecutive and follow a protocol order established for any manufacturer.
“The fastest vaccine ever”
Of the nearly 100 proposals for SARS-CoV-2 vaccines under development by research teams at private companies, research centers, universities and laboratories throughout the world, researchers have studied different techniques, some of which have not been used in licensed vaccines in the past.
At least nine groups have already started human safety trials according to WHO records , testing injectable vaccine formulations, while others are currently testing in animal models. Importantly, “running” companies have accelerated the vaccine development process in different ways: testing multiple animal models at the same time, and in parallel with testing small groups of people. Usually the process is to start in animal models and with these results advance to human trials, to ensure that a measurable immune response is obtained and that side effects are minimal. For this reason the researchers recognize that lack of time carries greater risk [13] .
However, since studies have shown that SARS-CoV-2 does not mutate as fast as other viruses such as influenza or HIV, there is confidence that a vaccine, once developed, can be effective and provide protection. for quite some time [14] . Since the development of a vaccine for COVID-19 has taken the path of “accelerated development” (Figure 2), Dan Barouch, director of Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, a professor at Harvard University School of Medicine, and leader of one of the laboratories working on a vaccine for COVID-19, notes that according to industry predictions in early 2021, could have an emergency or compassionate vaccine.
Barouch recalls that the Ebola vaccine, which also caused an emergency, took 5 years to reach the population [15] . An eventual COVID-19 vaccine is expected within a year, so the researcher concludes that “will be the fastest vaccine development in history” [16] .
Among the best known and rated as promising cases are the companies Moderna Therapeutics in Cambridge, Massachusetts, and Inovio Pharmaceuticals in Pennsylvania, both in the United States, which are conducting trials preclinical and clinical in parallel.
Moderna announced on May 18 that phase 1 human trials yielded promising results, however it is important to analyze this type of news critically . The company reported that the test consisted of inoculating only 8 healthy volunteers and measuring their immune response through the production of antibodies, which immunology experts rate as insufficient to guarantee the effectiveness [ 17] . With these results, and in conjunction with the U.S. National Institute of Allergy and Infectious Diseases, Moderna advanced to phase 2 of their trials.
The vaccine in development in the UK, led by Oxford University and the AstraZeneca company, which is in charge of licensing, received nearly 1.2 billion dollars from the US government [18] and is being committed for September this year [19] . Likewise, the American company Novavax is conducting clinical trials for the COVID-19 vaccine in Australia, and received a substantial contribution of $ 388 million from a non-profit initiative founded by Bill Gates [20] . This vaccine, if approved, would have a bit of Chile since its developers are currently investigating the use of saponin, a substance with a structure similar to the soap produced by the Quillay tree, native to our country, as an indispensable component of the vaccine enhancer, called “Matrix-M” [21] .
It is not a speed race
While a quick solution is needed, urgency cannot compromise people’s safety and health . The analogies of “the race for the coronavirus vaccine” exclude an inherent aspect of scientific work, which is testing a hypothesis based on concrete results, arising from exhaustive, rational and justified experimentation.
The accelerated development protocol ensures that the efficacy, safety, and quality of the final product that is a vaccine are not compromised. Adapting to normal development times exposes another aspect of scientific research: is not an individual exercise, but requires communication, cooperation and coordination between people, institutions and countries . Only in this way has it been possible to reformulate the development of a vaccine for COVID-19 and offer a solution in record time.
Developing a vaccine is not a speed race, it is rather a team sport or a human pyramid , in which each level and each block are essential to build the complete pyramid, from the base to the the top.
Not all vaccines are created equal
Currently, numerous coordinated and distributed human teams around the world are testing at least 8 types of vaccines for the new SARS-CoV-2 coronavirus. Among the types of vaccines in which they work, they use different approaches to fulfill the same purpose: in some cases they use the whole virus and in others they use parts of the virus to activate the immune response (Figure 7).
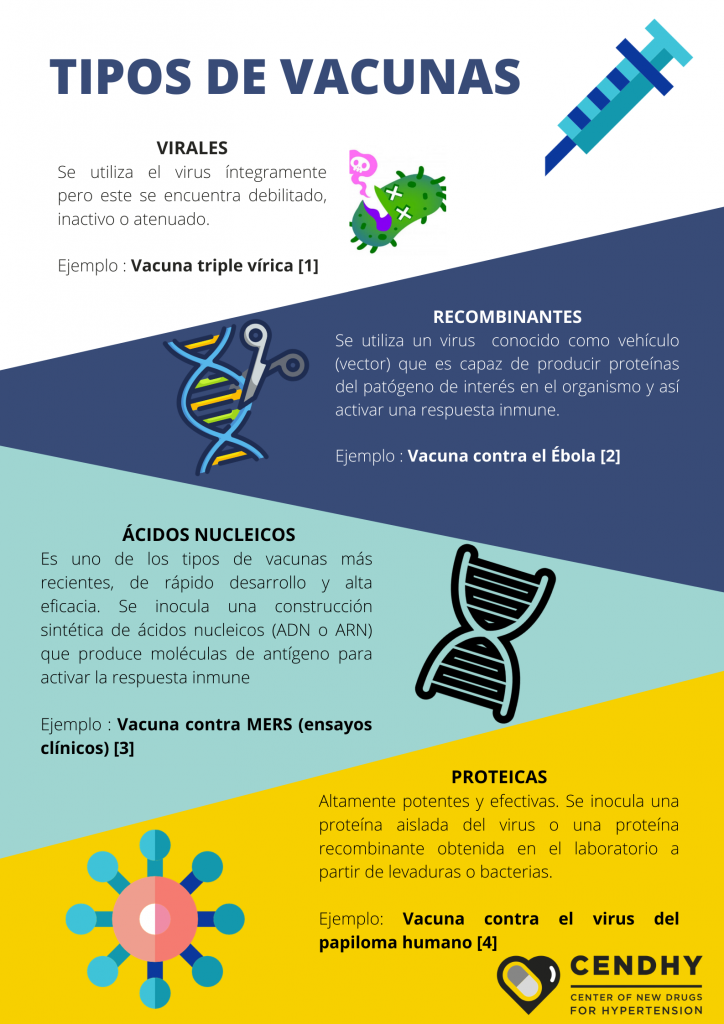
According to the press and in the words of the researchers themselves, the vaccines that “lead the way” in this speed race metaphor are basically the most advanced and have presented preliminary results in their human trials. These include the Moderna Therapeutics candidate who is a viral type of RNA ; the joint proposal between pharmaceutical Pfizer and the company BioNTech which is also RNA-based , while the strategy chosen by the company Inovio Pharmaceuticals is based on DNA [23] , like the Harvard University that works in association with Janssen Pharmaceutical of the multinational Johnson & Johnson [24] . The latest contender originating from the United States is that proposed by the biotech company Novavax and funded in part by Bill Gates.
The anticipated English promise from Oxford University and AstraZeneca would be of the recombinant type, as is the Ebola vaccine. Of this same nature is the vaccine under development at the CanSino Biologics company in China. Its direct local competition is 3 viral vaccines that use inactivated SARS-CoV-2, and are in development at the Wuhan Institute of Biological Products , the Beijing Institute of Biological Products and pharmaceutical Sinovac Biotech . Since the latter, the leaders of the research assured in late May that their CoronaVac vaccine has a 99% chance of being successful [25] after passing the trials in monkeys and advance to the second stage with more than 1000 human volunteers.
According to the most recent update, 10 candidates in the clinical stage are reported [26], most of them carrying out phases 1 and 2 in parallel (Figure 8).

The need over uncertainty
To conclude, it is important to understand that vaccines can be developed using different techniques, but the end is the same: to create a vaccine capable of generating immunity in the population to face this pandemic. Although this process can normally take more than 10 years, in this case of urgency to defeat COVID-19, both experimental and regulatory stages are being accelerated.
With accelerated processes such as these it is normal for doubts and mistrust to arise : is it safe? When will the vaccine arrive? Do I need to be vaccinated if I had the disease? Who is immune? Can I catch it again or catch it ?. After a review of studies on common colds, some researchers initially assumed that those recovered from SARS-CoV-2 infection can stay protected from reinfection for a certain period of time, which lists them as “immune” . Regarding these assumptions, Michael Diamond, a viral immunologist at the University of Washington in Missouri, United States, states that they must be “supported by evidence” . Diamond also states that “we do not know much about the immunity of this particular virus” [14] , against which caution and patience must be exercised until certainty is obtained.
Another of the most frequent concerns is about how long the immunity granted by the vaccine would last . According to Stanley Perlman, a coronavirus virologist at the University of Iowa: “There is no good evidence to indicate lasting immunity.” In response to this question, Diamond hopes that more can be learned about the infection in animal and human studies, and that “perhaps this accelerated way of developing a vaccine is not the most efficient but may be the most convenient” [14] .
The immediate need is to prevent more deaths and serious disease conditions, stop the rise of the contagion curve and protect the population, and against this the consensus decision between experts from various scientific areas, specialists doctors and public health authorities, it is to act intelligently regarding uncertainties , opting for the accelerated development of vaccines in a context of interdisciplinary collaboration.
Authors:
Victoria Aguayo, thesis student at Drug Delivery Lab and student of Biochemistry, Universidad de Chile.
Lorena Díaz Hemard , Scientific and Communications Coordinator at CENDHY. Engineer in Molecular Biotechnology, Universidad de Chile.
Javier Morales Montecinos , PhD. Director and principal investigator at CENDHY. Director of Drug Delivery Lab and academic University of Chile.
References:
[1] Panorama de candidatos para la vacuna de COVID-19 [2 de junio 2020]. World Health Organization (WHO). Disponible en línea: https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines
[2] Manual sobre la vacuna del SIDA. AVAC, Segunda Edición: “Perspectivas Globales”, 2005. Available online: https://www.avac.org/resource/aids-vaccine-handbook-2nd-edition-global-perspectives
[3] Vacuna para paperas. WHO, updated en 2011. Available online: https://www.who.int/biologicals/vaccines/mumps/en/
[4] “ UC researchers lead the development of the Chilean coronavirus vaccine ” (March 3, 2020). Online news Pontificia Universidad Católica de Chile. Available at: https: //www.uc. cl / news / uc-researchers-lead-development-of-chilean-vaccine-against-the-coronavirus /
[5] “ Promising Chilean Coronavirus Vaccine Begins Pre-Clinical Trials ” (May 21, 2020). Magazine Que Pasa de La Tercera. Available online: https : //www.latercera.com/que-pasa/noticia/prometedora-vacuna-chilena-contra-el-coronavirus-inicia-pruebas-pre-clinicas/GR67EPSCMRE6VHVNISADFA47ZY/
[6] Real-time statistics COVID-19. Worldometers: https://www.worldometers.info/coronavirus/
[7] Status Report No. 1: New Coronavirus (2019-nCoV), January 21 (in English) [January 21, 2020]. QUIEN. Available online: https: //www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4
[8] New Coronavirus Action Plan COVID-19, Ministry of Health. Available online: https://www.minsal.cl/new-coronavirus-2019-ncov/
[9] National Immunization Program, Ministry of Health. Available online: https://www.minsal.cl/programa-nacional-de-immunizaciones/
10] Hoffmann et al. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor . Cell Vol. 181, p .: 271–280. Available online (PDF): https://doi.org/10.1016/j.cell.2020.02.052 a>.
[11] Tripathi A, Bankaitis VA (2017). Molecular Docking: From Lock and Key to Combination Lock. Journal of Molecular Medicine and Clinical Applications Vol. 2 (1). DOI: http://dx.doi.org/10.16966/2575-0305.106 (available online).
[12] Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter (2002). Molecular Biology of the Cell. Garland Science, 4ta edición.
[13] Callaway, E. (March 18, 2020). Coronavirus vaccines: five key questions as trials begin . Nature, 579 (7800), 481. Available online: https://www.nature.com/articles / d41586-020-00798-8
[14] Callaway, E. (April 28, 2020). The race for coronavirus vaccines: a graphical guide . Nature, 580 (7805), 576. Available online: https://www.nature.com/articles / d41586-020-01221-y
[15] Julie E. Ledgerwood et al. (2017). Chimpanzee Adenovirus Vector Ebola Vaccine. New England Journal of Medicine Vol. 376: 928-38. DOI: 10.1056/NEJMoa1410863. Disponible en línea (PDF): https://www.nejm.org/doi/pdf/10.1056/NEJMoa1410863
[16] Charles Schmidt (2020). Genetic Engineering Could Make a COVID-19 Vaccine in Months Rather Than Years. Scientific American June 2020 Issue. Available online: https://www.scientificamerican.com/article/genetic-engineering-could-make-a-covid-19-vaccine-in-months-rather-than-years1/
[17] Nsikan Akpan (29 de mayo, 2020). A COVID-19 vaccine has passed its first human trial. But is it the frontrunner? National Geographic Science Coronavirus coverage. Available online: https://www.nationalgeographic.com/science/2020/05/coronavirus-vaccine-passes-first-human-trial-but-is-it-frontrunner-cvd/
[18] David D. Kirkpatrick (3 de junio, 2020). $1.2 Billion From U.S. to Drugmaker to Pursue Coronavirus Vaccine. The New York Times. Available online: https://www.nytimes.com/2020/05/21/health/coronavirus-vaccine-astrazeneca.html
[19] Coronavirus: AstraZeneca ready to supply potential vaccine in September (21 de mayo, 2020). BBC News. Available online: https://www.bbc.com/news/business-52751661
[20] Sissi Cao (26 de mayo, 2020). Bill Gates Funds a Crucial COVID-19 Vaccine Human Trial, Merck Adds 2 Candidates. Observer. Available online: https://observer.com/2020/05/coronavirus-vaccine-update-novavax-merck-bill-gates-funding/
[21] Quillay: El árbol nativo de Chile con el que se están fabricando vacunas contra el COVID-19 (28 de mayo, 2020). Futuro 360. Available online: https://www.futuro360.com/data/quillay-arbol-nativo-chile-fabricando-vacunas-contra-covid-19_20200528/
[22] Tipos de coronavirus humanos (2020). National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Centers for Disease Control and Prevention. Actualizado al 15 de febrero, 2020. Available online: https://www.cdc.gov/coronavirus/types.html
[23] Olivia Willis (23 de mayo, 2020). Who’s leading the race? A guide to coronavirus vaccines in the pipeline. ABC Health & Wellbeing. Available online: https://www.abc.net.au/news/health/2020-05-24/coronavirus-vaccine-race/12277558
[24] Global race to a COVID-19 vaccine (abril 2020). Harvard News. Available online: https://news.harvard.edu/gazette/story/2020/04/harvards-coronavirus-vaccine-efforts/
[25] Pharmaceutical ensures that there is a 99% chance that its coronavirus vaccine will work (29 de mayo, 2020). Meganoticias. Available online: https://www.meganoticias.cl/calidad-de-vida/303205-vacuna-coronavirus-exito-sinovac-farmaceutica-china-1yz.html [26] Mullard, A (2020). COVID-19 vaccine development pipeline gears up. The Lancet, Vol. 395(10239), p: 1751-1752. Disponible en línea (PDF): https://doi.org/10.1016/S0140-6736(20)31252-6


0 Comments